Abstract

Background: In immune thrombocytopenia (ITP), immunoglobulin G (IgG) platelet autoantibodies accelerate platelet clearance and impair platelet production. IgG homeostasis is regulated by the neonatal Fc receptor (FcRn). Efgartigimod (EFG), a human IgG1 Fc-fragment, is a natural ligand of FcRn engineered to competitively bind to FcRn with high affinity and prevent recycling of endogenous IgG, but not albumin, thereby reducing IgG levels including IgG autoantibody levels.
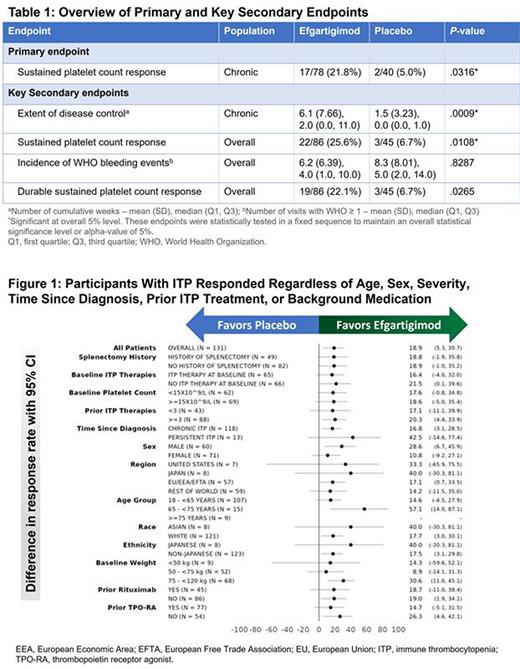
Methods: The efficacy and safety of intravenous (IV) EFG were evaluated in ADVANCE, a phase 3, multicenter, randomized, double-blinded, placebo (PBO)-controlled trial (NCT04188379) in adults with persistent or chronic ITP. Participants with an average of 2 platelet counts (PLTs) of <30×109/L during screening were randomized 2:1 to receive 10 mg/kg EFG or PBO for 24 weeks. Concurrent oral corticosteroids, oral immunosuppressants, dapsone, danazol, fostamatinib, and oral thrombopoietin receptor agonists (not romiplostim) were permitted but required to remain at the entry dosage and frequency. Participants received weekly dosing (weeks 1-4) followed by response-dependent weekly or every 2 weeks (q2w) dosing (weeks 5-16) and then maintained their dosing regimen from weeks 17-24. The primary and key secondary endpoints were tested in hierarchical order. The primary endpoint was the proportion of chronic ITP participants with a sustained PLT response (PLT of ≥50×109/L in ≥4 of 6 visits between weeks 19 and 24 without intercurrent events, e.g., rescue therapy at week 12 or later). Key secondary endpoints included extent of disease control (cumulative weeks with PLTs of ≥50×109/L) in the chronic population, proportion of participants in the overall population (chronic and persistent) with sustained PLT response, incidence of bleeding events, and a durable sustained platelet response (PLT of ≥50×109/L in ≥6 of 8 visits between weeks 17 and 24) in the overall population. Safety and pharmacodynamic measures were also evaluated.
Results: 131 participants (118 chronic ITP, 13 persistent ITP; 86 EFG, 45 PBO) were randomized. Participants had long-standing, severe ITP (median time since diagnosis of 4.57 years; baseline median PLT of 17×109/L) and were heavily pretreated (67.2% had ≥3 prior ITP therapies). In chronic ITP participants, sustained PLT response was reached in more EFG-treated participants (21.8%; 17/78) than PBO (5.0%; 2/40; p=0.0316). EFG achieved all PLT-related secondary endpoints (Table1). Model-based mean changes from baseline in PLT levels over time showed a clear differentiation from PBO as of week 1, indicating an early onset of PLT response in the EFG group. A sustained PLT response was achieved in 90% (9/10) of participants who reached and maintained the q2w fixed dosing. EFG-treated participants responded better than PBO regardless of age, sex, severity of disease, time since diagnosis, prior ITP treatment, or background medication (Figure1). International working group (IWG) response (consecutive visits ≥7 days apart with PLTs of ≥30×109/L and 2-fold increase from baseline in the absence of bleeding events) was seen in 51.2% (44/86) of EFG treated participants compared to 20.0% (9/45) in the PBO group.
Mean IgG levels in EFG-treated participants decreased steadily over the first 4 weeks of treatment, after which the mean maximum reductions from baseline remained greater than 60% throughout the trial. Mean IgG levels were similar in the group on q2w treatment. Adverse events (AEs) were reported in 93.0% (80/86) of participants in the EFG group and 95.6% (43/45) in the PBO group. Most frequent AEs included bruising, headache, hematuria, and petechiae. Serious AEs were reported in 8.1% (7/86) of participants in the EFG group and in 15.6% (7/45) in the PBO group, and none were treatment related. These included bleeding or events related to bleeding (n=5), infections (n=4), and worsening primary ITP (n=3). No deaths or increased risk of infection were observed.
Conclusions: Compared to PBO, EFG showed an early PLT increase, higher sustained PLT response, and more weeks with PLT ≥50×109/L. EFG achieved the primary and all PLT-related secondary endpoints and also showed that 51.2% of participants on EFG achieved IWG response criteria versus 20% on PBO. EFG was well tolerated with no new safety signals. Long-term efficacy and safety data are being evaluated in the open-label extension trial (ADVANCE+; NCT04225156).
Disclosures
Broome:Alexion, argenx, Apellis, Sanofi: Honoraria. McDonald:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: travel to conferences, Speakers Bureau; Sobi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Grifols: Research Funding; argenx: Other: travel to conferences; Rigel: Research Funding. Miyakawa:argenx, Kyowa Kirin, UCB, Zenyaku Kogyo: Consultancy; Alexion, Chugai, Pfizer, Sanofi: Honoraria. Carpenedo:Amgen, argenx, Novartis: Membership on an entity's Board of Directors or advisory committees; Sobi: Honoraria. Kuter:BioCryst: Consultancy, Research Funding; Dova: Consultancy; Kyowa Kirin: Consultancy; Incyte: Consultancy; Platelet Disorder Support Association: Consultancy; Sanofi: Consultancy; Shionogi: Consultancy; Shire: Consultancy; Up-To-Date: Consultancy; Zafgen: Consultancy; Rubius: Current holder of stock options in a privately-held company; Platelet Biogenesis: Consultancy; Cellularity: Consultancy; Cellphire: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Merck Sharp & Dohme: Consultancy; Momenta: Consultancy; Protalex: Consultancy, Research Funding; Caremark: Consultancy; Rigel: Consultancy, Research Funding; Takeda (Bioverativ): Consultancy, Research Funding; UCB: Consultancy, Research Funding; Principia: Consultancy, Research Funding; Kezar: Research Funding; Immunovant: Consultancy, Research Funding; argenx: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Genzyme: Consultancy; Alnylam: Consultancy, Research Funding; Actelion (Syntimmune): Consultancy, Research Funding; Agios: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; CRICO: Consultancy; Amgen: Consultancy, Research Funding; Hengrui: Consultancy. Al-Samkari:argenx: Consultancy; Sobi: Consultancy, Research Funding; Amgen: Research Funding; Novartis: Consultancy; Rigel: Consultancy; Forma: Consultancy; Moderna: Consultancy; Agios: Consultancy, Research Funding; Dova: Consultancy, Research Funding. Bussel:Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sobi: Consultancy, Membership on an entity's Board of Directors or advisory committees; UCB: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Data and Safety Monitoring Board; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Rallybio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Argenx: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Other: Data and Safety Monitoring Board; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Godar:argenx: Current Employment. Ayguasanosa:argenx: Current Employment. De Beuf:argenx: Current Employment. Rodeghiero:argenx, Novartis, Amgen, UCB: Consultancy, Membership on an entity's Board of Directors or advisory committees. Michel:UCB: Other: Boards, speaker at educational sessions; Sobi: Other: Boards, speaker at educational sessions; Novartis: Other: Boards, speaker at educational sessions; argenx: Other: Boards, speaker at educational sessions; Amgen: Other: Boards, speaker at educational sessions. Newland:Amgen: Consultancy, Honoraria, Research Funding; Angle: Consultancy, Honoraria; argenx: Consultancy, Honoraria; Dova: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Ono: Consultancy, Honoraria; Rigel: Consultancy, Honoraria, Research Funding; Shionogi: Consultancy, Honoraria.
OffLabel Disclosure:
Efgartigimod is not currently approved by any regulatory agency for the treatment of primary immune thrombocytopenia (ITP). This is the first report of topline results from a phase 3 trial evaluating the efficacy and safety of efgartigimod in primary ITP. Since efgartigimod, a human IgG1 fragment, is a natural ligand of FcRn, it prevents recycling of endogenous IgG antibodies, including platelet autoantibodies in the case of primary ITP.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal